 IN THIS TOPIC WE ARE GOING TO COVER FOLLOWING CONTENT
IN THIS TOPIC WE ARE GOING TO COVER FOLLOWING CONTENT 1)DISCOVERY OF SUBATOMIC PARTICLES
2)PROPERTIES OF SUBATOMIC PARTICLES
3)THOMSON AND RUTHERFORDS ATOMIC MODELS
4)PLANK'S QUANTUM THEORY
5)ELECTROMAGNETIC SPECTRA OF HYDROGEN ATOM
6)HEISENBERG'S UNCERTAINTY PRINCIPLE
7)IMPORTANT FORMULAE FOR EXAM
1)DISCOVERY OF SUBATOMIC PARTICLES:-
*ELECTRONS:-
In 1830, Michael Faraday showed that if
electricity is passed through a solution of an
electrolyte, chemical reactions occurred at the
electrodes, which resulted in the liberation
and deposition of matter at the electrodes. this matter deposited was electron. Its existance was proved using cathode ray tube experiment.
The results of these experiments are
summarised below.
(i) The cathode rays start from cathode and
move towards the anode.
(ii) These rays themselves are not visible but
their behaviour can be observed with the
help of certain kind of materials
(fluorescent or phosphorescent) which
glow when hit by them. Television
picture tubes are cathode ray tubes and
television pictures result due to
fluorescence on the television screen
coated with certain fluorescent or
phosphorescent materials.(iii) In the absence of electrical or magnetic
field, these rays travel in straight lines
(Fig. 2.2).
(iv) In the presence of electrical or magnetic
field, the behaviour of cathode rays are
similar to that expected from negatively
charged particles, suggesting that the
cathode rays consist of negatively
charged particles, called electrons.
(v) The characteristics of cathode rays
(electrons) do not depend upon the
material of electrodes and the nature of
the gas present in the cathode ray tube.
Thus, we can conclude that electrons are
basic constituent of all the atoms.
*PROTNS & NEUTRONS:-
Electrical discharge carried out in the
modified cathode ray tube led to the discovery
of particles carrying positive charge, also
known as canal rays. The characteristics of
these positively charged particles are listed
below.
(i) unlike cathode rays, the positively
charged particles depend upon the
nature of gas present in the cathode ray
tube. These are simply the positively
charged gaseous ions.
(ii) The charge to mass ratio of the particles
is found to depend on the gas from which
these originate.
(iii) Some of the positively charged particles
carry a multiple of the fundamental unit
of electrical charge.
(iv) The behaviour of these particles in the
magnetic or electrical field is opposite to
that observed for electron or cathode
rays.
The smallest and lightest positive ion was
obtained from hydrogen and was called
proton. This positively charged particle was
characterised in 1919. Later, a need was felt
for the presence of electrically neutral particle
as one of the constituent of atom. These
particles were discovered by Chadwick (1932)
by bombarding a thin sheet of beryllium by
α-particles. When electrically neutral particles
having a mass slightly greater than that of
the protons was emitted. He named these
particles as neutr ons.
2)PROPERTIES OF SUBATOMIC PARTICLES:-
3)THOMSON AND RUTHERFORDS ATOMIC MODELS:-
J. J. Thomson, in 1898, proposed that an
atom possesses a spherical shape (radius
approximately 10–10 m) in which the positive
charge is uniformly distributed. The electrons
are embedded into it in such a manner as to
give the most stable electrostatic arrangement
(Fig. 2.4). Many different names are given to
this model, for example, plum pudding,
raisin pudding or watermelon.
Rutherford and his students (Hans Geiger and
Ernest Marsden) bombarded very thin gold
foil with α–particles.. It was observed
that :
(i) most of the α– particles passed through
the gold foil undeflected.
(ii) a small fraction of the α–particles was
deflected by small angles.
(iii) a very few α– particles (∼1 in 20,000)
bounced back, that is, were deflected by
nearly 180°
.
4)PLANK'S QUANTUM THEORY:-
Some of the experimental phenomenon such
as diffraction* and interference** can be
explained by the wave nature of the
electromagnetic radiation. However, following
are some of the observations which could not
be explained with the help of even the
electromagentic theory of 19th century
physics (known as classical physics):
(i) the nature of emission of radiation from
hot bodies (black -body radiation)
(ii) ejection of electrons from metal surface
when radiation strikes it (photoelectric
effect)
(iii) variation of heat capacity of solids as a
function of temperature
(iv) line spectra of atoms with special
reference to hydrogen.
5)ELECTROMAGNETIC SPECTRA OF HYDROGEN ATOM:-
In the year 1885, on the basis of experimental observations, Balmer proposed the formula for correlating the wave number of the spectral lines emitted and the energy shells involved. This formula is given as:
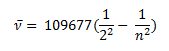
- Transition from the first shell to any other shell – Lyman series
- Transition from the second shell to any other shell – Balmer series
- Transition from the third shell to any other shell – Paschen series
- Transition from the fourth shell to any other shell – Bracket series
- Transition from the fifth shell to any other shell – Pfund series
6)HEISENBERG'S UNCERTAINTY PRINCIPLE:-
Werner Heisenberg a German physicist in
1927, stated uncertainty principle which is
the consequence of dual behaviour of matter
and radiation. It states that it is impossible
to determine simultaneously, the exact
position and exact momentum (or velocity)
of an electron.
7)IMPORTANT FORMULAE FOR EXAM:- 1. Velocity of electron in nth orbit = vn = 2.165 x 106 Z/n m/s
2. Radius of nth orbit = rn = 0.53 x 10–10 n2/Z m
3. Binding energy of an electron in nth state = En = –13.6 Z2/n2 eV/atom
En = –2.17 × 10–16 Zn2/n2 J/atom = –13.6 Zn2/n2 eV/atom
4. Kinetic energy = KE = 1/2 mv2n = KZe2 / rn
5. Potential energy = PE = –kZe2 / 2rn
6. Total energy of an electron = –En = –kZe2 / 2rn
PE = 2TE ; PE = –2KE ; TE = –KE
7. Binding energy of an electron in nth state
En = –13.6 / n2 Z2 eV
8. Ionisation Energy = – B.E.
I.E. = + 13.6 / n2 Z2 eV
9. Ionisation Potential
Ionisation potential = I.E. / e = 13.6/n2 Z2 V
10. Excitation Energy
The energy taken up by an electron to move from lower energy level to higher energy level. Generally it defined from ground state.
• Ist excitation energy = transition from n1 = 1 to n2 = 2
• IInd excitation energy = transition from n1 = 1 to n2 = 3
• IIIrd excitation energy = transition from n1 = 1 to n2 = 4 and so on …
• The energy level n = 2 is also called as Ist excited state.
• The energy level n = 3 is also called as IInd excited state. & so on …
In general, excitation energy (ΔE) when an electron is excited from a lower state n1 to any higher state n2 is given as:
ΔE = 13.6 Z2 (1/n12 – 1/n22) eV
11. Energy released when an electron jumps from a higher energy level (n2) to a lower energy level (n1) is given as:
ΔE = 13.6 Z2 (1/n12 – 1/n22) eV
If v be the frequency of photon emitted and λ be the wavelength, then:
ΔE =hv = h c/λ
The wavelength (λ) of the light emitted an also be determined by using:
1/λ = v = R Z2 (1/n12 – 1/n22)
R = 1.096 x 107 /m
Important: Also remember the value of 1/R = 911.5 Å for calculation of λ to be used in objectives only).
12. The number of spectral lines when an electron falls from n2 to n1 = 1 (i.e. to the ground state) is given by:
No. of lines = n2(n2–1) / 2
If the electron falls from n2 to n1, then the number of spectral lines is given by:
No. of lines = (n2 – n1 + 1) (n2–n1) / 2
HOPE THIS CONTENT HELPS.
😇😇 BE HAPPY , SPREAD SMILES😇😇





Comments
Post a Comment